Newest Findings
Presentation From the 66th American Society of Hematology (ASH) Annual Meeting & Exposition 2025
IONA-MM Second Interim Analysis
Real-World Experience with Isatuximab in Patients with Relapsed and/or Refractory Multiple Myeloma (RRMM)
Recently published data: Richardson PG et al. Haematologica. 2025.
ICARIA-MM Final Overall Survival Analysis
SARCLISA + Pd demonstrated a clinically meaningful OS benefit vs Pd
The addition of SARCLISA to Pd improved mOS by 6.9 months at a median follow-up of ~52 months

aOne-sided P value. Significance level is set to 0.02.
Adapted from Richardson PG et al. Haematologica. 2025. doi:10.3324/haematol.2023.284325
- SARCLISA + Pd improved median time to next treatment at 15.5 months (95% CI: 12.1, 19.8) vs Pd at 8.9 months (95% CI: 6.3, 11.5), HR=0.548 (95% CI: 0.417, 0.718)
- The addition of SARCLISA to Pd increased Grade ≥3 TEAE rates (91%) and serious TEAE rates (74%) vs Pd (76% and 61%, respectively) but did not increase fatal events or events leading to treatment discontinuation. No new safety concerns were identified with longer follow-up
HR=hazard ratio; mOS=median overall survival; OS=overall survival; Pd=pomalidomide and dexamethasone; TEAE=treatment-emergent adverse event.
Reference: Richardson PG, Perrot A, Miguel JS, et al. Isatuximab-pomalidomide-dexamethasone versus pomalidomide-dexamethasone in patients with relapsed and refractory multiple myeloma: final overall survival analysis. Haematologica. 2025. doi:10.3324/haematol.2023.284325
For more information about the phase 3 ICARIA-MM study, view SARCLISA + Pd Trial Design.

Presentations From the 20th International Myeloma Society (IMS) 2023 Annual Meeting and Exposition
IKEMA Final Overall Survival Analysis
SARCLISA + Kd demonstrated a clinically meaningful OS benefit vs Kd at a median follow-up of 56.6 months
*138 OS events: 79 (44.1%) in SARCLISA + Kd; 59 (48.0%) in Kd (cutoff date for OS analysis February 7, 2023). Results of a sensitivity analysis showed that COVID-19 disproportionately impacted OS in the SARCLISA + Kd arm; HR=0.803 (95% CI: 0.564, 1.142).
†Nominal one-sided P value.
Adapted from Yong K et al. Presented at: 20th International Myeloma Society (IMS) Annual Meeting and Exposition; September 27-30, 2023; Athens, Greece. ID #OA-48.
-
Safety summary at the OS analysis (median follow-up of 56.6 months):
- Grade ≥3 TEAEs and serious TEAEs occurred in 84.2% and 73.0%, and 71.2% and 60.7% of patients in the SARCLISA + Kd and Kd arms, respectively
- TEAEs leading to discontinuation occurred in 13.6% and 18.0% of patients in the SARCLISA + Kd and Kd arms, respectively
- Despite an additional 30 weeks of treatment exposure in the SARCLISA + Kd arm, the incidence of cardiac failure (grouping using MedDRA SMQ cardiac failure narrow terms) was similar in both arms: 8.5% (4.5% Grade ≥3) and 8.2% (4.1% Grade ≥3) in the SARCLISA + Kd and Kd arms, respectively
HR=hazard ratio; Kd=carfilzomib and dexamethasone; MedDRA=Medical Dictionary for Regulatory Activities; mOS=median overall survival; mPFS2=median second progression-free survival; mTTNT=median time to next treatment; NR=not reached; OS=overall survival; PFS2=second progression-free survival; SMQ=Standardised MedDRA Queries; TEAE=treatment-emergent adverse event.
Reference: Yong K, Martin T, Dimopoulos MA, et al. Isatuximab plus carfilzomib and dexamethasone versus carfilzomib and dexamethasone in patients with relapsed multiple myeloma (IKEMA): overall survival analysis. Presented at: 20th International Myeloma Society (IMS) Annual Meeting and Exposition; September 27-30, 2023; Athens, Greece. ID #OA-48.
For more information about the phase 3 IKEMA study, view SARCLISA + Kd Trial Design.

IMAGE Subgroup Analysis: SARCLISA + Pd in Patients With RRMM in Real-Life Context in France
SARCLISA + Pd showed a PFS benefit in subgroups of interest
Median PFS in elderly patients (age ≥75 years) was similar to that seen in patients age <75 years
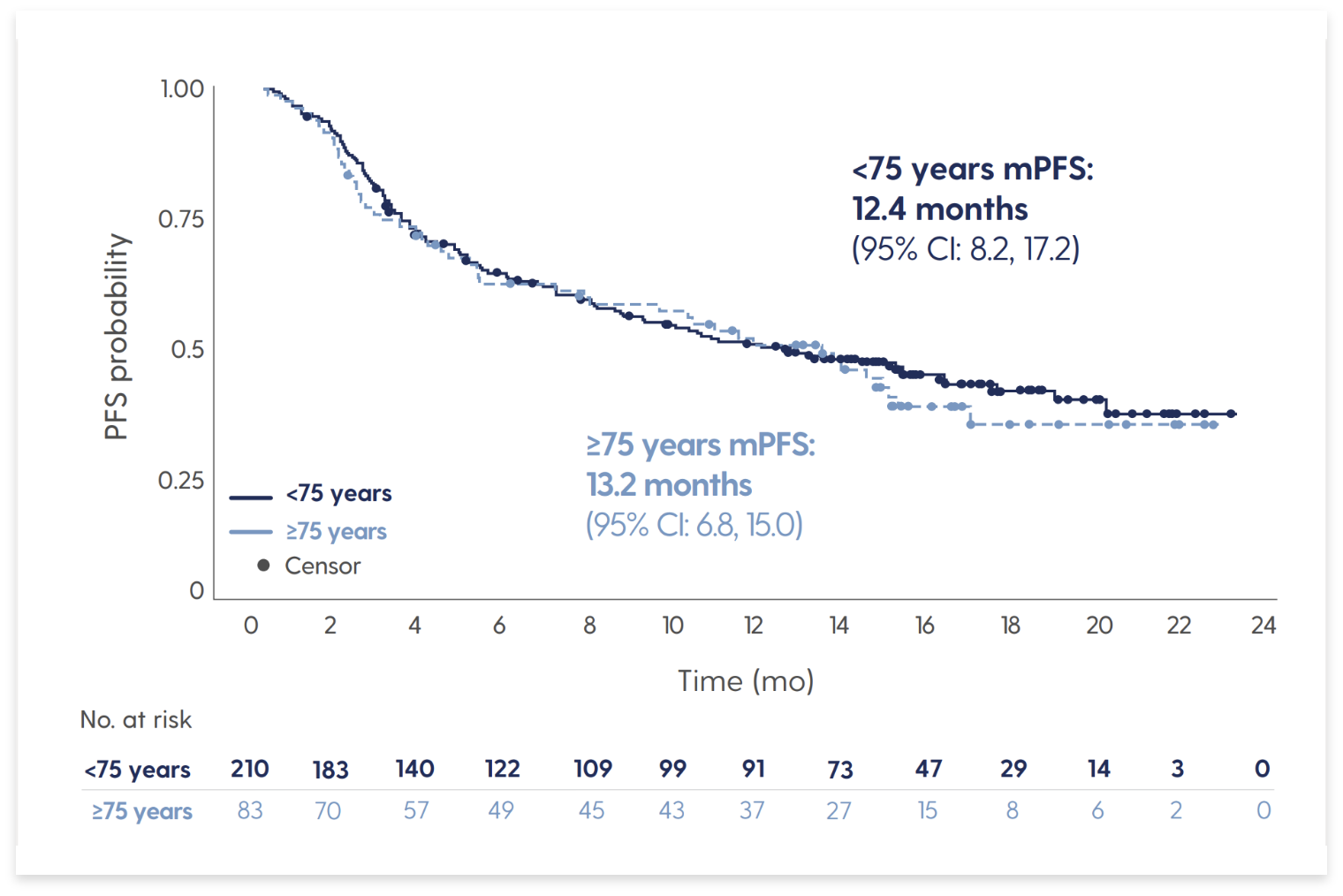
Adapted from Decaux O et al. Presented at: 20th International Myeloma Society (IMS) Annual Meeting and Exposition; September 27-30, 2023; Athens, Greece. Poster 287.
-
Results are from the noninterventional, retrospective IMAGE study conducted in France. In the overall study population (N=294):
- Patients had received a median of 2 prior lines of therapy (range 1-9)
- 73% of patients were refractory to lenalidomide
- Median PFS was 12.4 months, with a median follow-up of 14.2 months
-
Subgroups of interest included elderly patients (age ≥75 years; n=83), patients with severe renal impairment (eGFR <30 mL/min/1.73 m2; n=25), and patients with high-risk cytogenetics (presence of del[17p], t[4;14], or t[14;16]; n=40)
- Median PFS was 10.0 months in patients with eGFR <30 mL/min/1.73 m2 (95% CI: 2.4, 18.6) and 13.2 months in patients with eGFR ≥30 mL/min/1.73 m2 (95% CI: 9.0, 16.6)
- Median PFS was 7.6 months (95% CI: 2.8, NR) in patients with high-risk cytogenetics (n=40), 10.2 months (95% CI: 7.8, 14.2) in patients with standard-risk cytogenetics (n=134), and 15.0 months (95% CI: 11.2, 19.8) in patients whose cytogenetic risk was unknown (n=120)
- The data reported in the noninterventional IMAGE study are consistent with the benefit observed in the pivotal ICARIA-MM study
-
Safety summary (N=299):
- 3 elderly patients and 1 patient with high-risk cytogenetics had an AE leading to permanent discontinuation of SARCLISA
- Incidence of neutropenia in elderly patients, patients with severe renal impairment, and patients with high-risk cytogenetics was 12.0%, 0%, and 7.5%, respectively
- Infections and infestations occurred in 3 patients in the overall safety population: 2 who were elderly, 1 with severe renal impairment, and 0 with high-risk cytogenetics
AE=adverse event; eGFR=estimated glomerular filtration rate; mPFS=median progression-free survival; NR=not reached; Pd=pomalidomide and dexamethasone; PFS=progression-free survival; RRMM=relapsed and/or refractory multiple myeloma.
Reference: Decaux O, Fontan J, Perrot A, et al. Isatuximab plus pomalidomide and dexamethasone in patients with relapsed and/or refractory multiple myeloma in real-life context in France: IMAGE subgroup analysis based on subgroups of interest. Presented at: 20th International Myeloma Society (IMS) Annual Meeting and Exposition; September 27-30, 2023; Athens, Greece. Poster 287.
For more information about the phase 3 ICARIA-MM study, view SARCLISA + Pd Trial Design.

Presentations From the European Hematology Association (EHA) 2023 Hybrid Congress
SARCLISA in Relapsed Multiple Myeloma Patients With Ultra-High-Risk Cytogenetics
The addition of SARCLISA to Kd and Pd led to a PFS benefit regardless of risk category
PFS in IKEMA for patients with (A) standard risk, (B) extended high-risk, and (C) ultra-high-risk MM
PFS in ICARIA-MM for patients with (A) standard risk, (B) extended high-risk, and (C) ultra-high-risk MM
Adapted from Moreau P, et al. Presented at: European Hematology Association (EHA) 2023 Hybrid Congress; June 8-11, 2023; Frankfurt, Germany, and online.
- Extended high-risk was defined as the presence of one of the following: del(17p), t(4;14), t(14;16), or 1q21+, which includes both gain(1q21) and amp(1q21)
- Ultra-high-risk was defined as the presence of ≥2 of the following: del(17p), t(4;14), t(14;16), or 1q21+
- The incidence of grade ≥3 TEAEs was generally higher in the SARCLISA-containing arm than in the control arm in both trials, regardless of risk category, with the exception of standard-risk patients in IKEMA
- This information is from a post hoc analysis of the IKEMA and ICARIA-MM trials.
HR=hazard ratio; Kd=carfilzomib and dexamethasone; MM=multiple myeloma; mPFS=median progression-free survival; NR=not reached; Pd=pomalidomide and dexamethasone; PFS=progression-free survival; TEAE=treatment-emergent adverse event.
Reference: Moreau P, Perrot A, Dimopolous MA, et al. Isatuximab in relapsed multiple myeloma patients with ultra-high-risk cytogenetics: ICARIA-MM and IKEMA subgroup analysis. Presented at: European Hematology Association (EHA) 2023 Hybrid Congress; June 8-11, 2023; Frankfurt, Germany, and online. Poster P931.
For more information about the phase 3 SARCLISA studies, view SARCLISA + Kd Trial Design and SARCLISA + Pd Trial Design.

Long-Term Outcomes With SARCLISA + Kd in Relapsed Multiple Myeloma Patients With 1q21+ Status: Updated Results From the Phase 3 IKEMA Study
The addition of SARCLISA to Kd resulted in longer PFS compared to Kd in patients with 1q21+ chromosomal abnormalities
PFS in IKEMA in patients (A) without 1q21+, (B) with 1q21+, (C) with isolated 1q21+, (D) with gain(1q21), (E) with amp(1q21)
Adapted from Facon T, et al. Presented at: European Hematology Association (EHA) 2023
Hybrid Congress; June 8-11, 2023; Frankfurt, Germany, and online.
- The presence of 1q21+ chromosomal abnormalities (gain or amplification) is associated with a higher risk of progression and worse prognosis in patients with MM
-
In this updated subgroup analysis, PFS and depth of response was evaluated in relapsed MM patients with 1q21+ status. In the SARCLISA + Kd and Kd arms, respectively:
- 41.9% and 42.3% of patients had 1q21+ (≥3 copies, with or without HRCA)
- 26.3% and 25.2% of patients had isolated 1q21+ (≥3 copies, without HRCA)
- 24.0% and 30.1% of patients had gain(1q21) (3 copies, with or without HRCA)
- 17.9% and 12.2% of patients had amp(1q21) (≥4 copies, with or without HRCA)
- Treatment discontinuation rates due to adverse events were 10.7% and 13.1% in the SARCLISA + Kd arm vs 11.5% and 23.6% for the Kd arm in patients with 1q21+ and without 1q21+, respectively
HRCA=high-risk chromosomal abnormalities; Kd=carfilzomib and dexamethasone; MM=multiple myeloma; mPFS=median progression-free survival; NC=not calculable; PFS=progression-free survival.
Reference: Facon T, Moreau P, Spicka I, et al. Long-term outcomes with isatuximab-carfilzomib-dexamethasone (Isa-Kd) in relapsed multiple myeloma patients with 1q21+ status: updated results from the phase 3 IKEMA study. Presented at: European Hematology Association (EHA) 2023 Hybrid Congress; June 8-11, 2023; Frankfurt, Germany, and online. Poster P916.
For more information about the phase 3 SARCLISA studies, view SARCLISA + Kd Trial Design.

Allocation and Validation of the Second Revision of the International Staging System (R2-ISS) in the IKEMA and ICARIA-MM Studies
A study validating the R2-ISS in patients with RRMM and in patients treated with an anti-CD38 mAb
PFS by R2-ISS stage (pooled data from IKEMA and ICARIA-MM)

Adapted from Perrot A, et al. Presented at: European Hematology Association (EHA) 2023 Hybrid Congress; June 8-11, 2023; Frankfurt, Germany, and online.
- Pooled patients from both arms of IKEMA and ICARIA-MM were re-allocated into R2-ISS stage using the scoring strategy outlined by D’Agostino et al
- In this pooled analysis, SARCLISA triplet therapies (in combination with Kd and Pd) led to longer PFS vs comparator doublets regardless of R2-ISS stage
- In this study, PFS decreased with increasing R2-ISS stage, consistent with published findings
- In the IKEMA and ICARIA-MM trials (N=609), 68 patients were R2-ISS Stage I, 136 patients were Stage II, 204 patients were Stage III, 55 patients were Stage IV, and 146 patients were unclassified
Kd=carfilzomib and dexamethasone; mAb=monoclonal antibody; NC=not calculable; Pd=pomalidomide and dexamethasone;
PFS=progression-free survival; RRMM=relapsed or refractory multiple myeloma.
Reference: Perrot A, Richardson P, Mikhael J, et al. Allocation and validation of the second revision of the International Staging System in the ICARIA-MM and IKEMA studies. Presented at: European Hematology Association (EHA) 2023 Hybrid Congress; June 8-11, 2023; Frankfurt, Germany, and online. Poster P968.
For more information about the phase 3 SARCLISA studies, view SARCLISA + Kd Trial Design and SARCLISA + Pd Trial Design.

Prior Findings
Presentations From the 64th American Society of Hematology (ASH) Annual Meeting & Exposition (2022)
IKEMA Post Hoc Subgroup Analysis by Number of Prior Lines of Therapy
SARCLISA + Kd improved PFS and depth of response in patients with RRMM, regardless of the number of prior lines of therapy
mPFS: patients with only 1 prior line of therapy
Adapted from Capra et al. Presented at: 64th American Society of Hematology (ASH) Annual Meeting & Exposition; December 10-13, 2022; New Orleans, LA. Poster 3176.
- In patients who received 1 prior line of therapy, mPFS was 38.2 months with SARCLISA + Kd and 28.2 months with Kd (HR=0.723; 95.4% CI: 0.442, 1.184)
- In patients who received >1 prior line of therapy, mPFS was 29.2 months with SARCLISA + Kd and 17.0 months with Kd (HR=0.452; 95.4% CI: 0.298, 0.686)
- In this study, PFS decreased with increasing R2-ISS stage, consistent with published findings
- The frequency of Grade ≥3 TEAEs were: 83.3% (SARCLISA + Kd) and 72.2% (Kd), 1 prior line; 83.8% (SARCLISA + Kd) and 73.5% (Kd), >1 prior line. Serious TEAEs occurred in 66.7% vs 51.9% of patients (1 prior line subgroup) and 72.7% vs 66.2% of patients (>1 prior line subgroup) with SARCLISA + Kd vs Kd, respectively
HR=hazard ratio; Kd=carfilzomib and dexamethasone; mPFS=median progression-free survival; NC=not calculable;
PFS=progression-free survival; R2-ISS=Second Revision of the International Staging System; RRMM=relapsed and/or refractory multiple myeloma; TEAE=treatment-emergent adverse event.
Reference: Capra M, Martin T, Moreau P, et al. Isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma: IKEMA subgroup analysis by number of prior lines of treatment. Presented at: 64th American Society of Hematology (ASH) Annual Meeting & Exposition; December 10-13, 2022; New Orleans, LA. Poster 3176.
For more information about the phase 3 IKEMA study, view SARCLISA + Kd Trial Design.

IKEMA Subgroup Analysis: Early vs Late Relapse
This analysis examined the efficacy and safety of SARCLISA + Kd vs Kd in patients who experienced early vs late relapse from prior treatment
-
This is a post hoc analysis with a median follow-up of 44 months; this analysis examined PFS, ORR, ≥VGPR, CR, MRD-, and OS
- Early relapse was defined as <12 months since initiation of the most recent line of treatment in patients with ≥2 prior lines of treatment, <18 months in patients with 1 prior line of treatment, and <12 months in patients with ASCT
- Late relapse was defined as ≥12 months since initiation of the most recent line of treatment in patients with ≥2 prior lines of treatment and ≥18 months in patients with 1 prior line of treatment
- See the results by downloading the congress presentation below
ASCT=autologous stem cell transplant; CR=complete response; Kd=carfilzomib and dexamethasone; MRD-=minimal residual disease negative/negativity; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; VGPR=very good partial response.
Reference: Facon T, Moreau P, Baker R, et al. Isatuximab plus carfilzomib and dexamethasone in pts with early versus late relapsed multiple myeloma: Ikema subgroup analysis. Presented at: 64th American Society of Hematology (ASH) Annual Meeting & Exposition; December 10-13, 2022; New Orleans, LA. Presentation 753.
For more information about the phase 3 IKEMA study, view SARCLISA + Kd Trial Design.

Presentations from International Myeloma Society (IMS) 2022
IKEMA Updated PFS Analysis
At a median follow-up of 44 months, SARCLISA + Kd demonstrated unprecedented mPFS of 3 years in a pivotal trial that includes lenalidomide-refractory patients
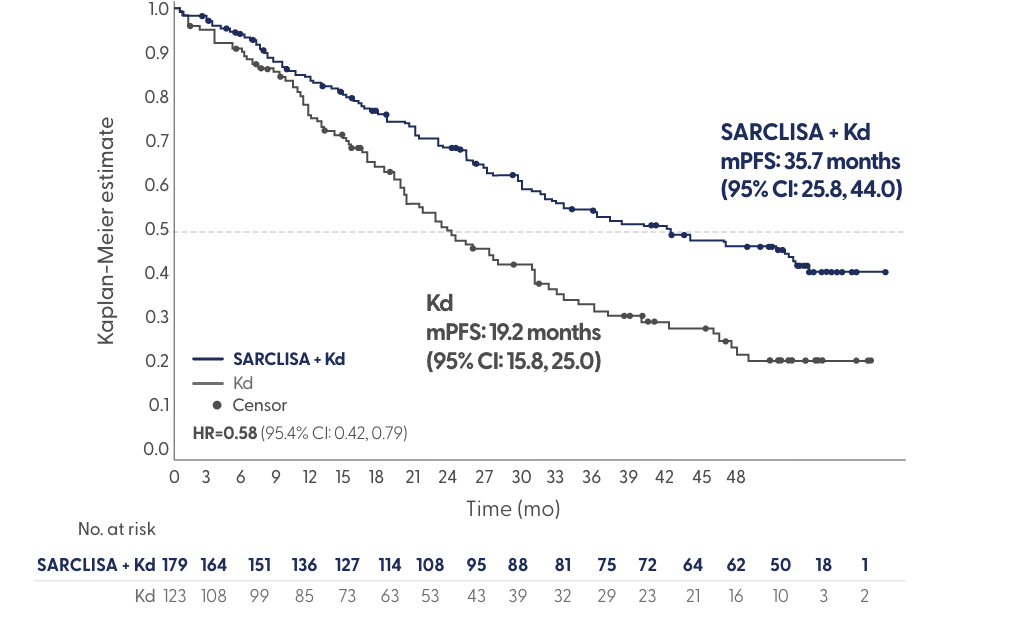
Adapted from Yong et al. Presented at: International Myeloma Society (IMS) Annual Meeting; August 25-27, 2022; Los Angeles, CA. Poster 284.
- At a median follow-up of 44 months, SARCLISA + Kd demonstrated deep responses: 44% CR and 34% MRD- (vs 29% and 15% with Kd, respectively)
- Safety profiles in both arms remained consistent with prior IKEMA findings; serious TEAEs were reported in 70% of patients on SARCLISA + Kd vs 60% on Kd
- The most common, non-haematologic TEAEs in the SARCLISA + Kd arm were: infusion reaction (46%), diarrhoea (40%), hypertension (38%), and upper respiratory tract infection (37%)
CR=complete response; HR=hazard ratio; Kd=carfilzomib and dexamethasone; mPFS=median progression-free survival; MRD-=minimal residual disease negative/negativity; PFS=progression-free survival; TEAE=treatment-emergent adverse event.
Reference: Yong K, Moreau P, Dimopoulos MA, et al. Updated progression-free survival and depth of response in IKEMA, a randomized phase 3 trial of isatuximab, carfilzomib and dexamethasone (Isa-Kd) vs Kd in relapsed multiple myeloma. Presented at: International Myeloma Society (IMS) Annual Meeting; August 25-27, 2022; Los Angeles, CA. Poster 284.
For more information about the phase 3 IKEMA study, view SARCLISA + Kd Trial Design.

IKEMA Updated Depth of Response Analysis
At a median follow-up of 44 months, SARCLISA + Kd demonstrated deep responses
*Stratified on randomisation factors according to IRT.
Adapted from Hajek et al. Presented at: International Myeloma Society (IMS) Annual Meeting; August 25-27, 2022; Los Angeles, CA. Presentation OAB-046.
- SARCLISA + Kd patients had a >2-fold higher likelihood of achieving MRD- compared to Kd patients
- The impact of achieving MRD- on long-term outcomes with SARCLISA + Kd vs Kd is discussed in the poster. MRD- rates seen in difficult-to-treat populations are also reported
- Grade ≥3 and serious TEAEs were higher in the SARCLISA + Kd arm, regardless of MRD status (85% MRD-, 83% MRD+) vs Kd (74% MRD-, 73% MRD+); however, the addition of SARCLISA to Kd did not increase fatal TEAEs or events leading to treatment discontinuation
CR=complete response; IRC=independent response committee; IRT=interactive response technology; Kd=carfilzomib and dexamethasone; MRD=minimal residual disease; MRD-=minimal residual disease negative/negativity; MRD+=minimal residual disease positive/positivity; NGS=next-generation sequencing; ORR=overall response rate; TEAE=treatment-emergent adverse event; VGPR=very good partial response.
Reference: Hajek R, Moreau P, Augustson B, et al. Depth of response of isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma: IKEMA updated analysis. Presented at: International Myeloma Society (IMS) Annual Meeting; August 25-27, 2022; Los Angeles, CA. Presentation OAB-046.
For more information about the phase 3 IKEMA study, view SARCLISA + Kd Trial Design.

ICARIA-MM Final Overall Survival Analysis
SARCLISA + Pd showed a clinically meaningful overall survival benefit, with a ~7-month median overall survival improvement vs Pd
SARCLISA + Pd showed a clinically meaningful overall survival benefit, with a ~7-month median overall survival improvement vs Pd
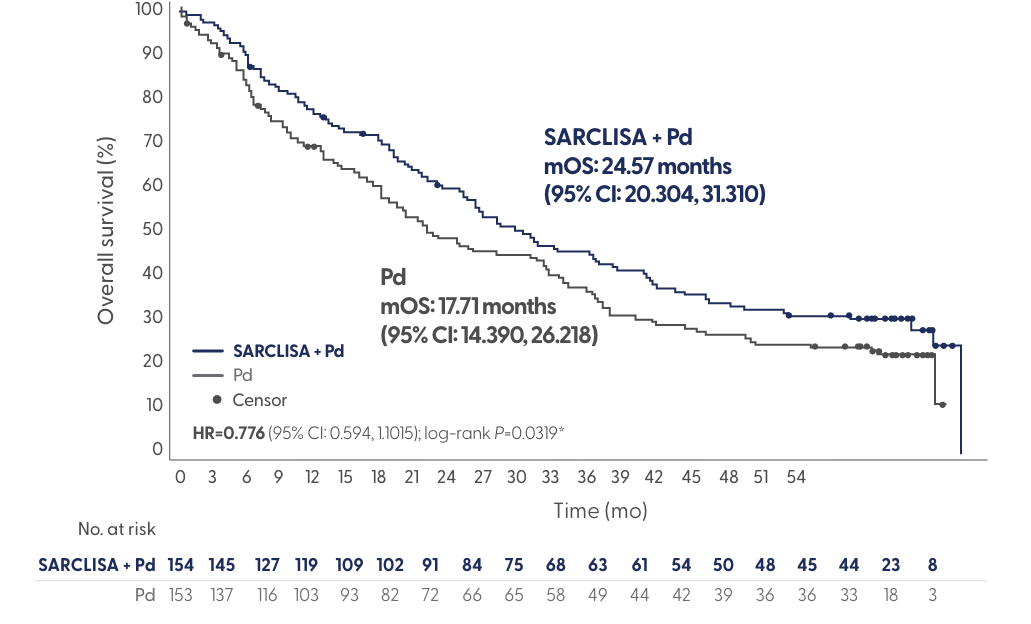
*One-sided P value, significance level is set to 0.02.
220 OS events: 106 (68.8%) in SARCLISA + Pd; 114 (74.5%) in Pd.
Adapted from Richardson et al. Presented at: International Myeloma Society (IMS) Annual Meeting; August 25-27, 2022; Los Angeles, CA. Presentation OAB-052.
- SARCLISA + Pd improved median time to next treatment at 15.51 months (95% CI: 12.123, 19.811) vs Pd at 8.87 months (95% CI: 6.341, 11.466), HR=0.548 (95% CI: 0.417, 0.718)
- The addition of SARCLISA to Pd increased Grade ≥3 TEAE rates (91%) and serious TEAE rates (74%) vs Pd (76% and 61% respectively), but did not increase fatal events or events leading to treatment discontinuation
HR=hazard ratio; mOS=median overall survival; mPFS=median progression-free survival; OS=overall survival; Pd=pomalidomide and dexamethasone; TEAE=treatment-emergent adverse event.
Reference: Richardson P, Perrot R, San-Miguel J, et al. Isatuximab plus pomalidomide/low-dose dexamethasone versus pomalidomide/low-dose dexamethasone in patients with relapsed/refractory multiple myeloma (ICARIA-MM): final overall survival analysis. Presented at: International Myeloma Society (IMS) Annual Meeting; August 25-27, 2022; Los Angeles, CA. Presentation OAB-052.
For more information about the phase 3 ICARIA-MM study, view SARCLISA + Pd Trial Design.




















